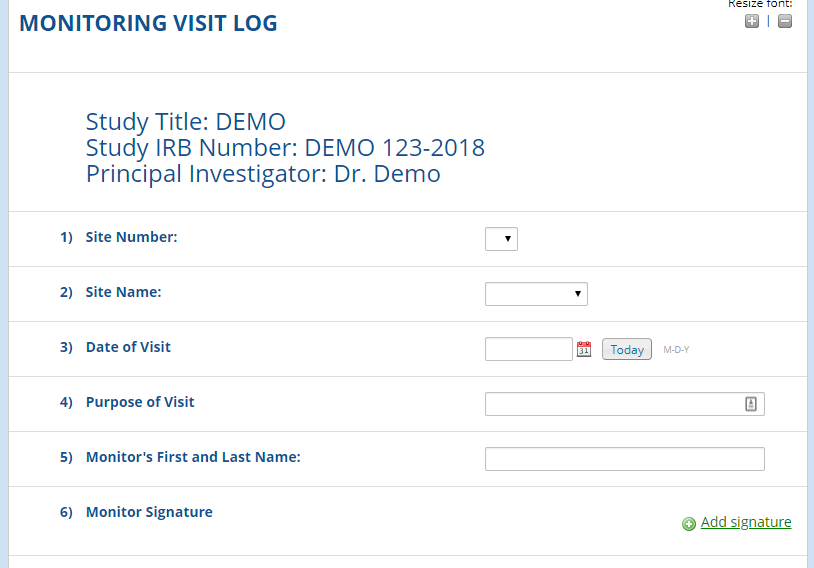
Monitoring logs are used to provide a comprehensive list of all monitoring visits. It is required for both observational and interventional clinical research studies.
Best Practice:
Record monitoring visits as they occur, to ensure completeness and accuracy of the data. If a visit occurs over more than one day, each day should be recorded separately.
The clinical site monitor should sign each line as a monitoring visit begins.
How to translate paper monitoring log to REDCap?
| Paper | REDCap feature |
|---|---|
| Descriptive Text Field |
| Descriptive Text Field |
| Multiple Choice (Dropdown or Radio) |
| Text box |
| Signature Field |
| Text box or Notes box |
| Text box with action tag @NOW & validation Date |
REDCap Example
Click MONITORING VISIT LOG for a demo. Feel free to text as many times as needed.
Download: Monitoring Visit Log Template
Developed by Theresa Baker
Overview
Content Tools